314-800-4742
1701 Quincy Ave. Suite 21 Naperville, IL 60540
Email: info@apt-therapeutics.com
Products
PRODUCTS
APT 102
Summary
APT102 represents a new paradigm for acute anti-thrombotic therapy; an approach that will decrease peri-procedural myocardial infarction and bleeding and thereby decrease long-term morbidity and mortality. APT102 is unique from current antiplatelet agents in structure, mechanism of action, anti-inflammatory and cardioprotective activity, and bleeding risk. It is expected to provide additional antithrombotic and cardioprotective efficacy when used alone, or as adjunctive therapy with other antithrombotic or thrombolytic agents, without increasing bleeding risk.
Product
APT102 is a recombinant form of human apyrase; optimized as an anti-platelet and anti-inflammatory agent with an undetectable risk of bleeding. APT102 is the only recombinant human apyrase currently available that is appropriate for pharmaceutical development. Preclinical pharmacology has identified the following key attributes of APT102:
- Strong antithrombotic efficacy
- No evidence of bleeding
- Anti-inflammatory efficacy
- Cardioprotection from reperfusion injury
- Rapid onset of action
- 20-40 hour pharmacodynamic half-life after a single i.v. or s.c. dose
Clinical Need
Dual anti-platelet inhibition with aspirin and clopidogrel has been shown to result in a reduction in cardiovascular events in a variety of settings. Attempts to improve outcomes further have led to the development of more potent antiplatelet agents, but at a cost of increased bleeding complications. Net clinical adverse outcomes, which incorporate adverse cardiovascular events and major bleeding, remain as high as 7-12% in patients treated for myocardial infarction. Furthermore, long-term morbidity and mortality are dramatically increased in patients experiencing peri-procedural myocardial infarction or bleeding. Consequently, the search for an fast-acting antiplatelet agent with optimal ischemic protection and an acceptable bleeding risk remains the 'holy grail' of antiplatelet drug development.
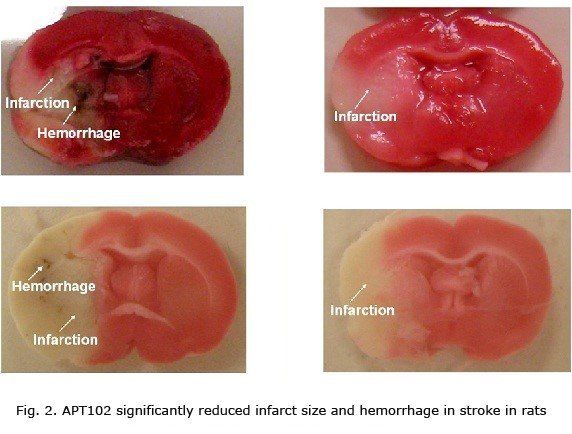
APT102 inhibits platelet activation and aggregation almost immediately upon i.v. administration and provides nearly complete platelet inhibition for at least 24 hr in dogs after a single bolus intravenous injection. APT102 demonstrated in vitro antiplatelet activity and in vivo antithrombotic efficacy in several well-characterized animal models of coronary thrombosis and stroke. Importantly, in every model evaluated, APT102 provided antithrombotic efficacy without increasing bleeding risk, even when administered in combination with other antithrombotic agents. APT102 also ameliorates ischemia/reperfusion injury and inflammatory response in several animal models, including lung transplantion.
Scientific publications
APT 102
- J S, Broekman M, Mitsky T, Chen R, Marcus A. Antithrombotic Activity of a Novel Engineered Human Apyrase. Enzymatic Profile, Ex Vivo and In Vivo Properties. Blood. 104: abstract 530, 2004. Oral presentation.
- Medina C, Jurasz P, Santos-Martinez MJ, J S, Mitsky T, Chen R.D. Radomski M. (2006). Platelet aggregation-induced by Caco-2 cells: Regulation by matrix metalloproteinase-2 and adenosine diphosphate. J. Pharmacol. Exp Ther. 317, 379-345.
- Uluckan O, Eagleton M, Floyd D, Morgan E, Hirbe AC, Kramer M, Dowland N, Prior JL, Piwnica-Worms, J S, Chen R, Weilbaecher K (2008). APT102, a novel ADPase cooperates with aspirin to disrupt bone metastasis in mice. J Cellular Biochem. 104: 1311-1323.
- Sugimoto S, Lin X, Lai J, Okazaki M, Das N, Li W, Krupnick A, Chen R, J S, Patterson GA, Kreisel D, Gelman AD (2009). Apyrase treatment prevents ischemia-reperfusion injury in rat lung isografts. J Thorac Cardiovasc Surg. 138: 752-759, 2009.
- Wang C, Shabanzadeh AP, He C, Pegg CC. APT102 protects the ischemically injured brain in rats. Neuroscience 2010 meeting. Nov 14. Oral presentation.
- Guanghua Sun, Xiurong Zhao, James Grotta, Sean Savitz, C Chen, Jaroslaw Aronowski. Apyrase, APT102, improves the rt-PA beneficial effect in thromboembolic stroke model. American Heart Association International stroke conference. Feb 9, 2011. Poster presentation.
- Douglas Moeckel, Pamela Baum, Annie Nguyen, J S, R Chen, Dana Abendschein. A novel human apyrase prevents reocclusion and substantially decreases infarct size without bleeding prolongation after coronary fibrinolysis in dogs. Arteriosclerosis, thrombosis and vascular biology 2011 scientific sessions of American Heart Association. April 28, 2011. Oral presentation.
- Ibrahim M, Wang X, Puyo CA, Montecalvo A, Huang HJ, Hachem RR, Andreetti C, Menna C, Chen R, Krupnick AS, Kreisel D, Rendina EA, Gelman AE. Human recombinant apyrase therapy protects against canine pulmonary ischemia-reperfusion injury. Heart Lung Transplant. 2014 (in press).
- Moeckel D, Jeong SS, Sun X, Broekman MJ, Nguyen A, Drosopoulos JH, Marcus AJ, Robson SC, Chen R, Abendschein D. Optimizing human apyrase to treat arterial thrombosis and limit reperfusion injury without increasing bleeding risk. SciTransl Med. 248:248ra105. 2014.
- Tan Z, Li X, Turner RC, Logsdon AF, Lucke-Wold B, DiPasquale K, Jeong SS, Chen R, Huber JD, Rosen CL. Combination treatment of r-tPA and an optimized human apyrase reduces mortality rate and hemorrhagic transformation 6h after ischemic stroke in aged female rats. Eur J Pharmacol. 738:368-73. 2014.
- Marcus AJ, Safier LB, Hajjar KA, Ullman HL, Islam N, Eiroa AM. Inhibition of platelet function by an aspirin-insensitive endothelial cell ADPase. Thromboregulation by endothelial cells. J. Clin Invest. 88: 1690-1696, 1991.
- Handa M, Guidotti. Purification and cloning of a soluble ATP-diphosphohydrolase (apyrase) from potato tubers (Solanum tuberosum) Biochem Biophys Res Commun 218: 916-923, 1996.
- Maliszewski CR et al. The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization J Immunol 153: 3574-3583, 1994.
- Wang TF and Guidotti G, CD39 is an ecto-(Ca2+,Mg2+)-apyrase. J.Biol.Chem. 271: 9898-9901, 1996.
- Marcus AJ, Broekman MJ, Drosopoulos JHF, Islam N, Alyonycheva TN, Safler LB, Hajjar KA, Posnett DN, Schoenborn MA, Schooley KA, Gayle, RB, Maliszewski CR. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest. 99: 1351-1360, 1997.
- Gayle RB, III, Maliszewski CR, Gimpel SD, Schoenborn MA, Caspary RG, Richards C, Brasel K, Price V, Drosopoulos JH, Islam N, Alyonycheva TN, Broekman MJ, and Marcus AJ, Inhibition of platelet function by recombinant soluble ecto-ADPase/CD39. J.Clin.Invest 101: 1851-1859, 1998.
- Marcus AJ, Broekman MJ, Drosopoulos JHF, Islam N, Pinsky DJ, Sesti C, Levi R. Roles of CD39 (NTPDase-1) in thromboregulation, cerebroprotection, and cardioprotection. Semin Thromb Hemost. 31: 234-246, 2005.
- Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemast. 31: 217-233, 2005.
- Bours MJL et al. Adenosine 5'-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 112, 358-404, 2006.
- Quigley E. The therapeutic potential of CD39: interview with Dr. Simon Robson. Expert Opin Ther Targets. 10: 649-652, 2006.
- Enjyoji K et al. Targeted disruption of cd39/ATP diphosphohydrolase results indisordered hemostasis and thromboregulation. Nat Med. 5: 1011-1017, 1999.
- Candinas D, Koyamada N, Miyatake T, Slegel J, Hancock W, Bach FH, Robson SC. Loss of rat glomerular ATP diphosphohydrolase activity during reperfusion injury is associated with oxidative stress reactions. Thromb Haemos. 76: 807-812, 1996.
- Robson S, Sevigny J, Imai M, Enyoji K. Thromboregulatory potential of endothelial CD39/nucleotide triphosphate diphosphohydrolase: modulation of purinergic signaling inplatelets. Emerging Therapeutic Targets. 4: 155-171, 2000.
- Bakker W, Mui K, van Son W. Detection of glomerular ischemia in chronic graft failure by quantification of the glomerular ectonucleotidase and acto-ATPase. In: Vanduffel L., ed. Proceedings of the second international workshop on acto-ATPase and related ectonucleotidase. Maastricht, The Nertherlands: Shaker Publisheing BV. 44-49, 2000.
- Li Y, Csizmadia E, Sevigny J, Enjyoji K, Robson SC. CD39 (NTPDase1) modulates allograft rejection and cellular immune responses. Amer J Transplant 3(suppl): 545, 2003.
- Guckelberger O, Sun X, Sevigny J et al. Beneficial effects of CD39/ecto-nucleotide triphosphate diphosphohydrolase-1 in murine intestinal ischemia-reperfusion injury. Thromb Haemost 91: 576-586, 2004.
- Dwyer KM, Robson SC, Nandurkar MH, Campbell DJ, Gock H, Murray-Segal LJ, Fisicaro N, Mysore TB, Kaczmarek E, Cowan PJ, d'Apice AJF. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest. 113: 1440-1446, 2004.
- Imai M, Takigami K, Guckelberger O, Enjyoji K, Smith RN, Lin Y, Csizmadia E, Sevigny J, Rosenberg RD, Bach FH, Robson SC. Modulation of nucleotide triphosphate diphosphohydrolase-1 (NTPDase-1)/cd39 in xenograft rejection. Mol Med. 5: 743-752, 1999.
- Gangagharan SP, Imai M, Rhynharr KK et al. Targeting platelet aggregation: CD39 gene transfer augments nucleotide triphosphate diphosphohydrolase activity in injured rabbit arteries. Surgery. 130: 296-303, 2001.
- Koyamada N, Miyatake T, Candinas D et al., Apyrase administration prolongs discordant xenograft survival. Transplantation. 62: 1739-1743, 1996.
- Kohler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Muller CE, Eltzschig HK. Cd39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 116: 1784-1794, 2007.
- Drosopoulos JF et al. Human solCD39 inhibits injury-induced development of neointimal hyperplasia. Thromb Haemost 103: 426-431, 2010.
- Pinsky DJ, Broekman MJ, Peschon JJ, Stocking KL, Fujita T, Ramasamy R, Connolly ES, Jr., Huang J, Kiss S, Zhang Y, Choudhri TF, McTaggart RA, Liao H, Drosopoulos JH, Price VL, Marcus AJ, and Maliszewski CR, Elucidation of the thromboregulatory role of CD39/ectoapyrase in the ischemic brain. J.Clin.Invest 109: 1031-1040, 2002.
- Belayev L, Khoutorova L, Deisher TA, Belayev A et al. Neuroprotective effects of solCD39, a novel platelet aggregation inhibitor, on transient middle cerebral artery occlusion in rats. Stroke. 34: 758-763, 2003.
- Reutershan J et al. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 23: 473-482, 2009.
- Eckle T et al. Identification of ectonucletidases CD39 and CD73 in innate protection during acute lung injury. J Immunol 178: 8127-8137, 2007.
- Idzko M et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med 13: 913-919, 2007.
- Grenz A et al. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 21: 2863-2872, 2007.
- O'Brient JR, Etherington M, Jamieson S. Refractory state of platelet aggregation with major operations. Lancet. 2: 741-743.
- Ardlie NG et al. Influence of apyrase on stability of suspensions of washed rabbit platelets. Proc Soc Exp Biol Med 136: 1021-1023, 1971.
- Baurand A, Eckly A, Hechler B et al. Differential regulation and relocalization of the platelet P2Y receptors after activation: A way to avoid loss of hemostatic properties? Mol Pharm. 67: 721-733, 2005.
Copyright © All Rights Reserved.